Review of the condition Methicillin Resistant Staph Aureus (MRSA) long considered to be a hospital acquired infection, which is now found in communities of non-hospitalized persons. The show covers: 1) Mechanisms of antibiotic resistance 2) Oral antibiotic treatments and 3) Parenteral antibiotics for MRSA. Series: "UC Grand Rounds Series" [3/2008] [Health and Medicine] [Professional Medical Education] [Show ID: 13704]
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium responsible for several difficult-to-treat infections in humans. It is also called multidrug-resistant Staphylococcus aureus and oxacillin-resistant Staphylococcus aureus (ORSA). MRSA is any strain of Staphylococcus aureus that has developed, through the process of evolution, resistance to beta-lactam antibiotics, which include the penicillins (methicillin, dicloxacillin, nafcillin, oxacillin, etc.) and the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-sensitive Staphylococcus aureus, or MSSA. The evolution of such resistance does not cause the organism to be more intrinsically virulent than strains of Staphylococcus aureus that have no antibiotic resistance, but resistance does make MRSA infection more difficult to treat with standard types of antibiotics and thus more dangerous.
MRSA is especially troublesome in hospitals, prisons, schools, and nursing homes, where patients with open wounds, invasive devices, and weakened immune systems are at greater risk of infection than the general public.
S. aureus most commonly colonizes the anterior nares (the nostrils). The rest of the respiratory tract, open wounds, intravenous catheters, and the urinary tract are also potential sites for infection. Healthy individuals may carry MRSA asymptomatically for periods ranging from a few weeks to many years. Patients with compromised immune systems are at a significantly greater risk of symptomatic secondary infection.
In most patients, MRSA can be detected by swabbing the nostrils and isolating the bacteria found inside. Combined with extra sanitary measures for those in contact with infected patients, screening patients admitted to hospitals has been found to be effective in minimizing the spread of MRSA in hospitals in the United States,[1] Denmark, Finland, and the Netherlands.[2]
MRSA may progress substantially within 24–48 hours of initial topical symptoms. After 72 hours, MRSA can take hold in human tissues and eventually become resistant to treatment. The initial presentation of MRSA is small red bumps that resemble pimples, spider bites, or boils; they may be accompanied by fever and, occasionally, rashes. Within a few days, the bumps become larger and more painful; they eventually open into deep, pus-filled boils.[3] About 75 percent of community-associated (CA-) MRSA infections are localized to skin and soft tissue and usually can be treated effectively[citation needed]. But some CA-MRSA strains display enhanced virulence, spreading more rapidly and causing illness much more severe than traditional healthcare-associated (HA-) MRSA infections, and they can affect vital organs and lead to widespread infection (sepsis), toxic shock syndrome, and necrotizing ("flesh-eating") pneumonia. This is thought to be due to toxins carried by CA-MRSA strains, such as PVL and PSM, though PVL was recently found to not be a factor in a study by the National Institute of Allergy and Infectious Diseases (NIAID) at the NIH. It is not known why some healthy people develop CA-MRSA skin infections that are treatable while others infected with the same strain develop severe infections or die.[4]
The most common manifestations of CA-MRSA are skin infections, such as necrotizing fasciitis and pyomyositis (most commonly found in the tropics), necrotizing pneumonia, infective endocarditis (which affects the valves of the heart), and bone and joint infections.[5] CA-MRSA often results in abscess formation that requires incision and drainage. Before the spread of MRSA into the community, abscesses were not considered contagious, because it was assumed that infection required violation of skin integrity and the introduction of staphylococci from normal skin colonization. However, newly emerging CA-MRSA is transmissible (similar, but with very important differences) from Hospital-Associated MRSA. CA-MRSA is less likely than other forms of MRSA to cause cellulitis.
Risk factors
Some of the populations at risk:- People with weak immune systems (people living with HIV/AIDS, people living with lupus, cancer patients, transplant recipients, severe asthmatics, etc.)
- Diabetics[6]
- Intravenous drug users [7]
- Users of quinolone antibiotics[8]
- Young children[citation needed]
- The elderly[citation needed]
- College students living in dormitories[7]
- People staying or working in a health care facility for an extended period of time[7]
- People who spend time in coastal waters where MRSA is present, such as some beaches in Florida and the west coast of the United States[9][10]
- People who spend time in confined spaces with other people, including occupants of homeless shelters and warming centers, prison inmates, military recruits in basic training,[11] and individuals who spend considerable time in changerooms or gyms.[citation needed]
- Urban under-served[12]
- Indigenous populations, including Native Americans, Native Alaskans, and Australian Aboriginals[12]
- Veterinarians, Livestock handlers, and Pet owners[12]
Hospital patients
Many MRSA infections occur in hospitals and healthcare facilities, with a higher incidence rate in nursing homes or long-term care facilities. When infections occur in this manner it is known as healthcare acquired MRSA or HA-MRSA. These Rates of MRSA infection are also increased in hospitalized patients who are treated with quinolones. Healthcare provider-to-patient transfer is common, especially when healthcare providers move from patient to patient without performing necessary hand-washing techniques between patients.[8][13]Prison inmates, military recruits, and the homeless
Prisons, military barracks, and homeless shelters can be crowded and confined, and poor hygiene practices may proliferate, thus putting inhabitants at increased risk of contracting MRSA.[12] Cases of MRSA in such populations were first reported in the United States, and then in Canada. The earliest reports were made by the CDC in state prisons. Subsequently reports of a massive rise in skin and soft tissue infections were reported by the CDC in the Los Angeles County Jail system in 2001, and this has continued. Pan et al. reported on the changing epidemiology of MRSA skin infection in the San Francisco County Jail, noting the MRSA accounted for more than 70% of S. aureus infection in the jail by 2002. Lowy and colleagues reported on frequent MRSA skin infections in New York State Prisons. Two reports on inmates in Maryland have demonstrated frequent colonization with MRSA.In the news media hundreds of reports of MRSA outbreaks in prisons appeared between 2000 and 2008. For example, in February 2008, The Tulsa County Jail in the U.S. State of Oklahoma started treating an average of twelve Staphylococcus cases per month.[14] A report on skin and soft tissue infections in the Cook County Jail in Chicago in 2004–05 demonstrated that MRSA was the most common cause of these infections among cultured lesions and furthermore that few risk factors were more strongly associated with MRSA infections than infections caused by methicillin-susceptible S. aureus. In response to these and many other reports on MRSA infections among incarcerated and recently incarcerated persons, the Federal Bureau of Prisons has released guidelines for the management and control of the infections although few studies provide an evidence base for these guidelines.
People in contact with live food-producing animals
Cases of MRSA have increased in livestock animals. CC398 is a new clone of MRSA that has emerged in animals and is found in intensively reared production animals (primarily pigs, but also cattle and poultry), where it can be transmitted to humans. Though dangerous to humans, CC398 is often asymptomatic in food-producing animals.[15]A 2011 study reported 47% of the meat and poultry sold in surveyed U.S. grocery stores was contaminated with S. aureus and, of those, 52%—or 24.4% of the total—were resistant to at least three classes of antibiotics. "Now we need to determine what this means in terms of risk to the consumer," said Dr. Keim, a co-author of the paper.[16] Some samples of commercially sold meat products in Japan were also found to harbor MRSA strains.[17]
Athletes
In the United States, there have been increasing numbers of reports of outbreaks of MRSA colonization and infection through skin contact in locker rooms and gyms, even among healthy populations[citation needed]. A study published in the New England Journal of Medicine linked MRSA to the abrasions caused by artificial turf.[18] Three studies by the Texas State Department of Health found that the infection rate among football players was 16 times the national average. In October 2006, a high school football player was temporarily paralyzed from MRSA-infected turf burns. His infection returned in January 2007 and required three surgeries to remove infected tissue, as well as three weeks of hospital stay.[19]Children
MRSA is also becoming a problem in pediatric settings,[20] including hospital nurseries.[21] A 2007 study found that 4.6% of patients in U.S. health care facilities were infected or colonized with MRSA.[22]Diagnosis
Diagnostic microbiology laboratories and reference laboratories are key for identifying outbreaks of MRSA. New rapid techniques for the identification and characterization of MRSA have been developed. This notwithstanding, the bacterium generally must be cultured via blood, urine, sputum, or other body fluid cultures, and cultured in the lab in sufficient quantities to perform these confirmatory tests first. Consequently, there is no quick and easy method to diagnose a MRSA infection. Therefore, initial treatment is often based upon 'strong suspicion' by the treating physician, since any delay in treating this type of infection can have fatal consequences. These techniques include Real-time PCR and Quantitative PCR and are increasingly being employed in clinical laboratories for the rapid detection and identification of MRSA strains.[23][24]Another common laboratory test is a rapid latex agglutination test that detects the PBP2a protein. PBP2a is a variant penicillin-binding protein that imparts the ability of S. aureus to be resistant to oxacillin.[25]
Genetics
Antimicrobial resistance is genetically based; resistance is mediated by the acquisition of extrachromosomal genetic elements containing resistance genes. Exemplary are plasmids, transposable genetic elements, and genomic islands, which are transferred between bacteria via horizontal gene transfer.[26] A defining characteristic of MRSA is its ability to thrive in the presence of penicillin-like antibiotics, which normally prevent bacterial growth by inhibiting synthesis of cell wall material. This is due to a resistance gene, mecA, which stops β-lactam antibiotics from inactivating the enzymes (transpeptidases) that are critical for cell wall synthesis.SCCmec
Staphylococcal cassette chromosome mec (SCCmec) is a genomic island of unknown origin containing the antibiotic resistance gene mecA.[27][28] SCCmec contains additional genes beyond mecA, including the cytolysin gene psm-mec, which may suppress virulence in hospital-acquired MRSA strains.[29] SCCmec also contains ccrA and ccrB; both genes encode recombinases that mediate the site-specific integration and excision of the SCCmec element from the S. aureus chromosome.[27][28] Currently, six unique SCCmec types ranging in size from 21–67 kb have been identified;[27] they are designated types I-VI and are distinguished by variation in mec and ccr gene complexes.[26] Owing to the size of the SCCmec element and the constraints of horizontal gene transfer, a limited number of clones is thought to be responsible for the spread of MRSA infections.[27]Different SCCmec genotypes confer different microbiological characteristics, such as different antimicrobial resistance rates.[30] Different genotypes are also associated with different types of infections. Types I-III SCCmec are large elements that typically contain additional resistance genes and are characteristically isolated from HA-MRSA strains.[28][30] Conversely, CA-MRSA is associated with types IV and V, which are smaller and lack resistance genes other than mecA.[28][30]
mecA
mecA is responsible for resistance to methicillin and other β-lactam antibiotics. After acquisition of mecA, the gene must be integrated and localized in the S. aureus chromosome.[27] mecA encodes penicillin-binding protein 2a (PBP2a), which differs from other penicillin-binding proteins as its active site does not bind methicillin or other β-lactam antibiotics.[27] As such, PBP2a can continue to catalyze the transpeptidation reaction required for peptidoglycan cross-linking, enabling cell wall synthesis in the presence of antibiotics. As a consequence of the inability of PBP2a to interact with β-lactam moieties, acquisition of mecA confers resistance to all β-lactam antibiotics in addition to methicillin.[27]mecA is under the control of two regulatory genes, mecI and mecR1. MecI is usually bound to the mecA promoter and functions as a repressor.[26][28] In the presence of a β-lactam antibiotic, MecR1 initiates a signal transduction cascade that leads to transcriptional activation of mecA.[26][28] This is achieved by MecR1-mediated cleavage of MecI, which alleviates MecI repression.[26] mecA is further controlled by two co-repressors, BlaI and BlaR1. blaI and blaR1 are homologous to mecI and mecR1, respectively, and normally function as regulators of blaZ, which is responsible for penicillin resistance.[27][31] The DNA sequences bound by MecI and BlaI are identical;[27] therefore, BlaI can also bind the mecA operator to repress transcription of mecA.[31]
Strains
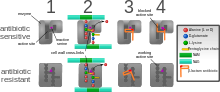
Diagram depicting antibiotic resistance through alteration of the
antibiotic's target site, modeled after MRSA's resistance to penicillin.
Beta-lactam antibiotics permanently inactivate PBP enzymes, which are essential for bacterial life, by permanently binding to their active sites. Some forms of MRSA, however, expresses a PBP that will not allow the antibiotic into its active site.
In the UK, where MRSA is commonly called "Golden Staph", the most common strains of MRSA are EMRSA15 and EMRSA16.[33] EMRSA16 is the best described epidemiologically: it originated in Kettering, England, and the full genomic sequence of this strain has been published.[34] EMRSA16 has been found to be identical to the ST36:USA200 strain, which circulates in the United States, and to carry the SCCmec type II, enterotoxin A and toxic shock syndrome toxin 1 genes.[35] Under the new international typing system, this strain is now called MRSA252. EMRSA 15 is also found to be one of the common MRSA strains in Asia. Other common strains include ST5:USA100 and EMRSA 1.[36] These strains are genetic characteristics of HA-MRSA.[37]
It is not entirely certain why some strains are highly transmissible and persistent in healthcare facilities.[32] One explanation is the characteristic pattern of antibiotic susceptibility. Both the EMRSA15 and EMRSA16 strains are resistant to erythromycin and ciprofloxacin. It is known that Staphylococcus aureus can survive intracellularly,[38] for example in the nasal mucosa [39] and in the tonsil tissue, .[40] Erythromycin and Ciprofloxacin are precisely the antibiotics that best penetrate intracellularly; it may be that these strains of S. aureus are therefore able to exploit an intracellular niche.
Community-acquired MRSA (CA-MRSA) strains emerged in late 1990 to 2000, infecting healthy people; who have not been in contact with health care facilities.[37] Researchers suggests that CA-MRSA did not evolved from the HA-MRSA.[37] This is further proven by molecular typing of CA-MRSA strains[41] and genome comparison between CA-MRSA and HA-MRSA, which indicate that novel MRSA strains integrated SCCmec into MSSA separately on its own.[37] By mid 2000, CA-MRSA was introduced into the health care systems and distinguishing between CA-MRSA from HA-MRSA became a difficult process.[37] Community-acquired MRSA (CA-MRSA) is more easily treated and more virulent, than hospital-acquired MRSA (HA-MRSA).[37] The genetic mechanism for the enhanced virulence in CA-MRSA remains as an active area of research. Especially, the Panton-Valentine leukocidin (PVL) genes are of interest because they are a unique feature of CA-MRSA.[32]
In the United States, most cases of CA-MRSA are caused by a CC8 strain designated ST8:USA300, which carries SCCmec type IV, Panton-Valentine leukocidin, PSM-alpha and enterotoxins Q and K,[35] and ST1:USA400.[42] ST8:USA300 strain results in skin infections, necrotizing fasciitis, toxic shock syndrome. Whereas, ST1:USA400 strain results in necrotizing pneumonia and pulmonary sepsis.[32] Other community-acquired strains of MRSA are ST8:USA500 and ST59:USA1000. In many nations of the world, MRSA strains with different predominant genetic background types have come to predominate among CA-MRSA strains; USA300 easily tops the list in the U. S. and is becoming more common in Canada after its first appearance there in 2004. For example, in Australia ST93 strains are common, while in continental Europe ST80 strains predominate (Tristan et al., Emerging Infectious Diseases, 2006), which carries SCCmec type IV.[43] In Taiwan, ST59 strains, some of which are resistant to many non-beta-lactam antibiotics, have arisen as common causes of skin and soft tissue infections in the community. In a remote region of Alaska, unlike most of the continental U. S., USA300 was found rarely in a study of MRSA strains from outbreaks in 1996 and 2000 as well as in surveillance from 2004–06 (David et al., Emerg Infect Dis 2008).
In June 2011, the discovery of a new strain of MRSA was announced by two separate teams of researchers in the UK. Its genetic make-up was reportedly more similar to strains found in animals, and testing kits designed to detect MRSA were unable to identify it.[44] This MRSA strain, Clonal Complex 398 (CC398), is responsible for Livestock-associated MRSA (LA-MRSA) infections.[36] Although it is known to be more persistent in colonizing pigs and calves, there has been cases of LA-MRSA carriers with pneumonia, endocarditis, and necrotising fasciitis.[45]
Prevention
Screening programs
Patient screening upon hospital admission, with nasal cultures, prevents the cohabitation of MRSA carriers with non-carriers, and exposure to infected surfaces. The test used (whether a rapid molecular method or traditional culture) is not as important as the implementation of active screening.[46] In the United States and Canada, the Centers for Disease Control and Prevention issued guidelines on October 19, 2006, citing the need for additional research, but declined to recommend such screening.[47][48]In some UK hospitals screening for MRSA is performed in every patient[49] and all NHS surgical patients, except for minor surgeries, are previously checked for MRSA.[50] There is no community screening in the UK, however screening of individuals is offered by some private companies.[51]
In a US cohort of 1300 healthy children, 2.4% carried MRSA in their nose.[52]
Surface sanitizing
Alcohol has been proven to be an effective surface sanitizer against MRSA. Quaternary ammonium can be used in conjunction with alcohol to extend the longevity of the sanitizing action.[citation needed] The prevention of nosocomial infections involves routine and terminal cleaning. Non-flammable Alcohol Vapor in Carbon Dioxide systems (NAV-CO2) do not corrode metals or plastics used in medical environments and do not contribute to antibacterial resistance.In healthcare environments, MRSA can survive on surfaces and fabrics, including privacy curtains or garments worn by care providers. Complete surface sanitation is necessary to eliminate MRSA in areas where patients are recovering from invasive procedures. Testing patients for MRSA upon admission, isolating MRSA-positive patients, decolonization of MRSA-positive patients, and terminal cleaning of patients' rooms and all other clinical areas they occupy is the current best practice protocol for nosocomial MRSA.
Studies published from 2004-2007 reported hydrogen peroxide vapor could be used to decontaminate busy hospital rooms, despite taking significantly longer than traditional cleaning. One study noted rapid recontamination by MRSA following the hydrogen peroxide application[53][54][55][56][57]
Also tested, in 2006, was a new type of surface cleaner, incorporating accelerated hydrogen peroxide, which was pronounced "a potential candidate" for use against the targeted microorganisms.[58]
Hand washing
In September 2004,[59] after a successful pilot scheme to tackle MRSA, the UK National Health Service announced its Clean Your Hands campaign. Wards were required to ensure that alcohol-based hand rubs are placed near all beds so that staff can hand wash more regularly. It is thought that even if this cuts infection by no more than 1%, the plan will pay for itself many times over.[citation needed]As with some other bacteria, MRSA is acquiring more resistance to some disinfectants and antiseptics. Although alcohol-based rubs remain somewhat effective, a more effective strategy is to wash hands with running water and an anti-microbial cleanser with persistent killing action, such as Chlorhexidine.[60] In another study chlorohexidine (Hibiclens), p-chloro-m-xylenol (Acute-Kare), hexaclorophene (Phisohex), and povidone-iodine (Betadine) were evaluated for their effectiveness. Of the four most commonly used antiseptics, povidone-iodine, when diluted 1:100, was the most rapidly bactericidal against both MRSA and methicillin-susceptible S. aureus.[61]
A June 2008 report[citation needed], centered on a survey by the Association for Professionals in Infection Control and Epidemiology, concluded that poor hygiene habits remain the principal barrier to significant reductions in the spread of MRSA.
Use of surgical respirator
The U.S. Food and Drug Administration (FDA) announced on 8 April 2011 that it had cleared a novel type of N95 Surgical Respirator, the SpectraShield 9500, that kills methicillin-resistant Staphylococcus aureus, Streptococcus pyogenes and Haemophilus influenzae. This mask is manufactured by Nexera Medical Ltd. of Richmond, British Columbia The mask blocks at least 95% of small particles in a standardized test. The FDA clearance also included evaluation by the National Institute of Occupational Safety and Health.[62]Proper disposal of hospital gowns
Used paper hospital gowns are associated with MRSA hospital infections, which could be avoided by proper disposal.[63]Isolation
Excluding medical facilities, current US guidance does not require workers with MRSA infections to be routinely excluded from the general workplace.[64] Therefore, unless directed by a health care provider, exclusion from work should be reserved for those with wound drainage that cannot be covered and contained with a clean, dry bandage and for those who cannot maintain good hygiene practices.[64] Workers with active infections should be excluded from activities where skin-to-skin contact is likely to occur until their infections are healed. Health care workers should follow the Centers for Disease Control and Prevention's Guidelines for Infection Control in Health Care Personnel.[65]To prevent the spread of staph or MRSA in the workplace, employers should ensure the availability of adequate facilities and supplies that encourage workers to practice good hygiene; that surface sanitizing in the workplace is followed; and that contaminated equipment are sanitized with Environmental Protection Agency (EPA)-registered disinfectants.[64]
Restricting antibiotic use
Glycopeptides, cephalosporins and in particular quinolones are associated with an increased risk of colonisation of MRSA. Reducing use of antibiotic classes that promote MRSA colonisation, especially fluoroquinolones, is recommended in current guidelines.[8][13]Public health considerations
Mathematical models describe one way in which a loss of infection control can occur after measures for screening and isolation seem to be effective for years, as happened in the UK. In the "search and destroy" strategy that was employed by all UK hospitals until the mid-1990s, all patients with MRSA were immediately isolated, and all staff were screened for MRSA and were prevented from working until they had completed a course of eradication therapy that was proven to work. Loss of control occurs because colonised patients are discharged back into the community and then readmitted; when the number of colonised patients in the community reaches a certain threshold, the "search and destroy" strategy is overwhelmed.[66] One of the few countries not to have been overwhelmed by MRSA is the Netherlands: An important part of the success of the Dutch strategy may have been to attempt eradication of carriage upon discharge from hospital.[67]The Centers for Disease Control and Prevention (CDC) estimated that about 1.7 million nosocomial infections occurred in the United States in 2002, with 99,000 associated deaths.[68] The estimated incidence is 4.5 nosocomial infections per 100 admissions, with direct costs (at 2004 prices) ranging from $10,500 (£5300, €8000 at 2006 rates) per case (for bloodstream, urinary tract, or respiratory infections in immunocompetent patients) to $111,000 (£57,000, €85,000) per case for antibiotic-resistant infections in the bloodstream in patients with transplants. With these numbers, conservative estimates of the total direct costs of nosocomial infections are above $17 billion. The reduction of such infections forms an important component of efforts to improve healthcare safety. (BMJ 2007)[citation needed] MRSA alone was associated with 8% of nosocomial infections reported to the CDC National Healthcare Safety Network from January 2006 to October 2007.[69]
This problem is not unique to one country; the British National Audit Office estimated that the incidence of nosocomial infections in Europe ranges from 4% to 10% of all hospital admissions. As of early 2005, the number of deaths in the United Kingdom attributed to MRSA has been estimated by various sources to lie in the area of 3,000 per year.[70] Staphylococcus bacteria account for almost half of all UK hospital infections. The issue of MRSA infections in hospitals has recently been a major political issue in the UK, playing a significant role in the debates over health policy in the United Kingdom general election held in 2005.
On January 6, 2008, half of 64 non-Chinese cases of MRSA infections in Hong Kong in 2007 were Filipino domestic helpers. Ho Pak-leung, professor of microbiology at the University of Hong Kong, traced the cause to high use of antibiotics. In 2007, there were 166 community cases in Hong Kong compared with 8,000 hospital-acquired MRSA case (155 recorded cases—91 involved Chinese locals, 33 Filipinos, 5 each for Americans and Indians, and 2 each from Nepal, Australia, Denmark and England).[71]
Worldwide, an estimated 2 billion people carry some form of S. aureus; of these, up to 53 million (2.7% of carriers) are thought to carry MRSA.[72] In the United States, 95 million carry S. aureus in their noses; of these, 2.5 million (2.6% of carriers) carry MRSA.[73] A population review conducted in three U.S. communities showed the annual incidence of CA-MRSA during 2001–2002 to be 18–25.7/100,000; most CA-MRSA isolates were associated with clinically relevant infections, and 23% of patients required hospitalization.[74]
One possible contribution to the increased spread of MRSA infections comes from the use of antibiotics in intensive pig farming. A 2008 study in Canada found MRSA in 10% of tested pork chops and ground pork; a U.S. study in the same year found MRSA in the noses of 70% of the tested farm pigs and in 45% of the tested pig farm workers.[75] There have also been anecdotal reports of increased MRSA infection rates in rural communities with pig farms.[76]
Healthcare facilities with high bed occupancy rates, high levels of temporary nursing staff, or low cleanliness scores no longer have significantly higher MRSA rates. Simple tabular evidence helps provide a clear picture of these changes, showing, for instance, that hospitals with occupancy over 90% had, in 2006–2007, MRSA rates little above those in hospitals with occupancy below 85%, in contrast to the period 2001–2004. In one sense, the disappearance of these relationships is puzzling. Reporters now blame IV cannula and catheters for spreading MRSA in hospitals. (Hospital organisation and speciality mix, 2008)[citation needed]
Decolonization
Care should be taken when trying to drain boils, as disruption of surrounding tissue can lead to larger infections, or even infection of the blood stream (often with fatal consequences).[77] Any drainage should be disposed of very carefully. After the drainage of boils or other treatment for MRSA, patients can shower at home using chlorhexidine (Hibiclens) or hexachlorophene (Phisohex) antiseptic soap (available over-the-counter at many pharmacies) from head to toe. Alternatively, a dilute bleach bath can be taken at a concentration of 1/2 cup bleach per 1/4-full bathtub of water.[78] Care should be taken to use a clean towel, and to ensure that nasal discharge (i.e. snot) doesn't infect the towel (see below).All infectious lesions should be kept covered with a dressing (band-aids etc.).[77] Mupirocin (Bactroban) 2% ointment can be effective at reducing the size of lesions. A secondary covering of clothing is preferred.[79] As shown in an animal study with diabetic mice, the topical application of a mixture of sugar (70%) and 3% povidone-iodine paste is an effective agent for the treatment of diabetic ulcers with MRSA infection.[80]
The nose is a common refuge for MRSA, and a test swab can be taken of the nose to indicate whether MRSA is present.[81] Mupirocin (Bactroban) If MRSA is detected via nasal culture, 2% ointment can be applied inside each nostril twice daily for 7 days, using a cotton-tipped swab. However, care should be taken so that the swab doesn't penetrate into the sinus. Household members are recommended to follow the same decolonization protocol. After treatment, the nose should be swabbed again to ensure that the treatment was effective. If not, the process should be repeated.
Toilet seats are a common vector for infection, and wiping seats clean before and/or after use can help to prevent the spread of MRSA. Door handles, faucets, light switches (with care!), etc. can be disinfected regularly with disinfectant wipes.[79] Spray disinfectants can be used on upholstery. Carpets can be washed with disinfectant, and hardwood floors can be scrubbed with diluted tea tree oil (e.g. Melaleuca). Laundry soap containing tea tree oil may be effective at decontaminating clothing and bedding, especially if hot water and heavy soil cycles are used, however tea tree oil may cause a rash which MRSA can re-colonize. Alcohol-based sanitizers can be placed near bedsides, near sitting areas, in vehicles etc. to encourage their use.
Doctors may also prescribe antibiotics such as clindamycin, doxycycline or trimethoprim/sulfamethoxazole.
Community settings
The CDC offers suggestions for preventing the contraction and spread MRSA infection which are applicable to those in community settings, including incarcerated populations, childcare center employees, and athletes. To prevent MRSA infection, individuals should regularly wash hands using soap and water or an alcohol-based sanitizer, keep wounds clean and covered, avoid contact with other people's wounds, avoid sharing personal items such as razors or towels, shower after exercising at athletic facilities (including gyms, weight rooms, and school facilities), shower before using swimming pools or whirlpools, and maintain a clean environment.[82]It may be difficult for people to maintain the necessary cleanliness if they do not have access to facilities such as public toilets with handwashing facilities. In the United Kingdom, the Workplace (Health, Safety and Welfare) Regulations 1992 requires businesses to provide toilets for their employees, along with washing facilities including soap or other suitable means of cleaning. Guidance on how many toilets to provide and what sort of washing facilities should be provided alongside them is given in the Workplace (Health, Safety and Welfare) Approved Code of Practice and Guidance L24, available from Health and Safety Executive Books. But there is no legal obligation on local authorities in the United Kingdom to provide public toilets, and although in 2008 the House of Commons Communities and Local Government Committee called for a duty on local authorities to develop a public toilet strategy [1] this was rejected by the Government [2].
Treatment
Both CA-MRSA and HA-MRSA are resistant to traditional anti-staphylococcal beta-lactam antibiotics, such as cephalexin. CA-MRSA has a greater spectrum of antimicrobial susceptibility, including to sulfa drugs (like co-trimoxazole/trimethoprim-sulfamethoxazole), tetracyclines (like doxycycline and minocycline) and clindamycin, but the drug of choice for treating CA-MRSA is now believed to be vancomycin, according to a Henry Ford Hospital Study. HA-MRSA is resistant even to these antibiotics and often is susceptible only to vancomycin. Newer drugs, such as linezolid (belonging to the newer oxazolidinones class) and daptomycin, are effective against both CA-MRSA and HA-MRSA. Linezolid is now felt to be the best drug for treating patients with MRSA pneumonia.[83] Ceftaroline and ceftabiparole, a new fifth generation cephalosporins, are the first beta-lactam antibiotics approved in the US to treat MRSA infections (skin and soft tissue only).[citation needed]Vancomycin and teicoplanin are glycopeptide antibiotics used to treat MRSA infections.[84] Teicoplanin is a structural congener of vancomycin that has a similar activity spectrum but a longer half-life.[85] Because the oral absorption of vancomycin and teicoplanin is very low, these agents must be administered intravenously to control systemic infections.[86] Treatment of MRSA infection with vancomycin can be complicated, due to its inconvenient route of administration. Moreover, many clinicians believe that the efficacy of vancomycin against MRSA is inferior to that of anti-staphylococcal beta-lactam antibiotics against methicillin-susceptible Staphylococcus aureus (MSSA).[87][88]
Several newly discovered strains of MRSA show antibiotic resistance even to vancomycin and teicoplanin. These new evolutions of the MRSA bacterium have been dubbed Vancomycin intermediate-resistant Staphylococcus aureus (VISA).[89] [90] Linezolid, quinupristin/dalfopristin, daptomycin, ceftaroline, and tigecycline are used to treat more severe infections that do not respond to glycopeptides such as vancomycin.[91]
There have been claims that bacteriophage can be used to cure MRSA.[92][93]
Cannabinoids, chemicals found in the Cannabis plant, are also suspected of being highly effective at killing MRSA.[94]
The psychedelic mushroom Psilocybe semilanceata has been shown to strongly inhibit the growth of Staphylococcus aureus.[95]
Initial studies at the University of East London have demonstrated that allicin (a compound found in garlic) exhibits a strong antimicrobial response to the bacteria, indicating that it may one day lead to more effective treatments.[96]
A report released in 2010 details the efficacy of the active ingredients of a new composite dressing (hydrogen peroxide, tobramycin, chlorhexidine digluconate, chlorhexidine gluconate, levofloxacin, and silver) against MRSA.[97]
A 1990 study tested MRSA isolates obtained from veterans and found they could be killed by several substances, including bacitracin, nitrofurantoin, hydrogen peroxide, novobiocin, netilmicin and vancomycin. The study went on to conclude that netilmicin might be useful as an alternative to intravenous vancomycin, and suggested that topical applications of hydrogen peroxide may be useful to reduce MRSA on skin and some mucous membranes.[98]
History
| The examples and perspective in this article may not represent a worldwide view of the subject. (December 2010) |
US and UK
In 1959 methicillin was licensed in England to treat penicillin-resistant S. aureus infections. Just as bacterial evolution had allowed microbes to develop resistance to penicillin, strains of S. aureus evolved to become resistant to methicillin. In 1961 the first MRSA isolates were reported in a British study, and between 1961-1967 there were infrequent hospital outbreaks in Western Europe and Australia.[99] The first United States hospital outbreak of MRSA occurred at the Boston City Hospital in 1968. Between 1968-mid 1990s the percent of S. aureus infections that were caused by MRSA increased steadily, and MRSA became recognized as an endemic pathogen. In 1974 2% of hospital-acquired S. aureus infections could be attributed to MRSA.[100] The rate had increased to 22% by 1995, and by 1997 the percent of hospital S. aureus infections attributable to MRSA had reached 50%.The first report of CA-MRSA occurred in 1981, and in 1982 there was a large outbreak of CA-MRSA among intravenous drug users in Detroit, Michigan.[99] Additional outbreaks of CA-MRSA were reported through the 1980s and 1990s, including outbreaks among Australian Aboriginal populations that had never been exposed to hospitals. In the mid 1990s there were scattered reports of CA-MRSA outbreaks among US children. While HA-MRSA rates stabilized between 1998–2008, CA-MRSA rates continued to rise. A report released by The University of Chicago Children's Hospital comparing two time periods (1993–1995 and 1995–1997) found a 25-fold increase in the rate of hospitalizations due to MRSA among children in the United States.[101] In 1999 The University of Chicago reported the first deaths from invasive MRSA among otherwise healthy children in the United States.[99] By 2004 MRSA accounted for 64% of hospital-acquired S. aureus infections in the United States.
The Office for National Statistics reported 1,629 MRSA-related deaths in England and Wales during 2005, indicating a MRSA-related mortality rate half the rate of that in the United States for 2005, even though the figures from the British source were explained to be high because of "improved levels of reporting, possibly brought about by the continued high public profile of the disease"[102] during the time of the 2005 United Kingdom General Election. MRSA is thought to have caused 1,652 deaths in 2006 in UK up from 51 in 1993.[103]
It has been argued that the observed increased mortality among MRSA-infected patients may be the result of the increased underlying morbidity of these patients. Several studies, however, including one by Blot and colleagues, that have adjusted for underlying disease still found MRSA bacteremia to have a higher attributable mortality than methicillin-susceptible S. aureus (MSSA) bacteremia.[104]
A population-based study of the incidence of MRSA infections in San Francisco during 2004–05 demonstrated that nearly 1 in 300 residents suffered from such an infection in the course of a year and that greater than 85% of these infections occurred outside of the healthcare setting.[105] A 2004 study showed that patients in the United States with S. aureus infection had, on average, three times the length of hospital stay (14.3 vs. 4.5 days), incurred three times the total cost ($48,824 vs $14,141), and experienced five times the risk of in-hospital death (11.2% vs 2.3%) than patients without this infection.[106] In a meta-analysis of 31 studies, Cosgrove et al.,[107] concluded that MRSA bacteremia is associated with increased mortality as compared with MSSA bacteremia (odds ratio = 1.93; 95% CI = 1.93±0.39).[108] In addition, Wyllie et al. report a death rate of 34% within 30 days among patients infected with MRSA, a rate similar to the death rate of 27% seen among MSSA-infected patients.[109]
According to the CDC, the most recent estimates of the incidence of healthcare-associated infections that are attributable to MRSA in the United States indicate a decline in such infection rates. Incidence of MRSA central line-associated blood stream infections as reported by hundreds of intensive care units decreased 50-70% from 2001-2007.[100] A separate system tracking all hospital MRSA bloodstream infections found an overall 34% decrease between 2005-2008.[100]
MRSA is sometimes sub-categorised as community-acquired MRSA (CA-MRSA) or healthcare-associated MRSA (HA-MRSA), although the distinction is complex. Some researchers have defined CA-MRSA by the characteristics of patients whom it infects, while others define it by the genetic characteristics of the bacteria themselves. By 2005, identified CA-MRSA risk factors included athletes, military recruits, incarcerated people, emergency room patients, urban children, HIV-positive individuals, men who have sex with men, and indigenous populations.[99]
Worldwide
The first reported cases of CA-MRSA began to appear in the mid-1990s in Australia, New Zealand, the United States, the United Kingdom, France, Finland, Canada and Samoa, and were notable because they involved people who had not been exposed to a healthcare setting.[5]Because measurement and reporting varies, it is difficult to compare rates of MRSA in different countries. An international comparison of 2004 MRSA-attributable S. arueus rates in middle and high income countries released by the Center For Disease Dynamics, Economics, and Policy in showed that Iceland had the lowest rate of infection, and Romania had the highest at over 70%.[110]
Research
Clinical
It has been reported that maggot therapy to clean out necrotic tissue of MRSA infection has been successful. Studies in diabetic patients reported significantly shorter treatment times than those achieved with standard treatments.[111][112][113]Many antibiotics against MRSA are in phase II and phase III clinical trials. e.g.:
- Phase III : ceftobiprole, Ceftaroline, Dalbavancin, Telavancin, Aurograb, torezolid, iclaprim etc.
- Phase II : nemonoxacin.[114]
Pre-clinical
An entirely different and promising approach is phage therapy (e.g., at the Eliava Institute in Georgia[115]), which in mice had a reported efficacy against up to 95% of tested Staphylococcus isolates.[116]On May 18, 2006, a report in Nature identified a new antibiotic, called platensimycin, that had demonstrated successful use against MRSA.[117][118]
A 2010 study noted significant antimicrobial action of Ulmo 90 and manuka UMF 25+ honey against several microorganisms, including MRSA. The investigators noted the superior antimicrobial action of Ulmo 90 honey, and suggested it be investigated further.[119] A separate 2010 study examined the use of medical-grade honey against several antibiotic-resistant strains of bacteria, including MRSA. The study concluded that the antimicrobial action of the honey studied was due to the activity of hydrogen peroxide, methylglyoxal, and a novel compound named bee defensin-1.[120]
Ocean-dwelling living sponges produce compounds that may make MRSA more susceptible to antibiotics.[121]
Some semi-toxic fungi/mushrooms excrete broad spectrum antibiotics, not all of which have been fully identified.[122]
Cannabinoids (components of Cannabis sativa), including cannabidiol (CBD), cannabinol (CBN), cannabichromene (CBC), tetrahydrocannabinol (THC) and cannabigerol (CBG), show activity against a variety of MRSA strains.[123]




No comments:
Post a Comment